LySAIRI: towards an innovative radiotherapy that facilitates immune sparing to improve overall clinical efficacy

Lymphocyte-Sparing Artificial Intelligence-guided Radio-Immunotherapy (LySAIRI) is an innovative large-scale project coordinated by Gustave Roussy and selected for funding by the French Hospital-University Health Research (RHU) action over the period 2022-2027. With his team, Prof. Eric Deutsch is establishing a new paradigm for improving radiotherapy techniques, notably by exploring strategies that aim at preserving the immune system of patients treated with radiation therappy.
It is estimated that more than half of all patients with cancer will receive radiotherapy at some point during their fight against the disease. Alone or combined with chemotherapy, it is the treatment of choice in many situations, whether curative or palliative, even if it does have limitations. For instance, by affecting the immune system, it can lead to lymphopenia, i.e. the drop of lymphocytes count (a type of white blood cells), which play an essential role in the anti-tumor immune response.
A paradigm shift in radioimmunotherapy
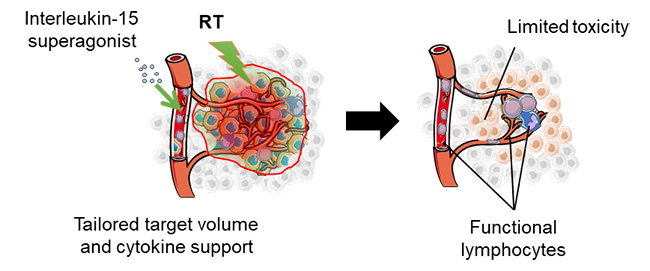
The idea behind the project is to achieve lymphocyte sparing and thus, to avoid so-called "radiation-induced lymphopenia", the severity of which is closely correlated with survival rates. Two main avenues are being explored. Firstly, radiotherapy prescriptions will be adapted to avoid, as far as possible, irradiating healthy tissues containing important quantities of lymphocytes. Secondly, a new immuno-radiotherapy combination will be evaluated, combining radiation with the pharmaceutical support of a drug.
The LySAIRI program aims to address these challenges through tailored, personalized and low-toxicity treatment planning, while effectively boosting the immune response via the holistic and synergistic action of radio-immunotherapy. "This is the first time that the interaction between radiotherapy and the immune system is been studied in this way. Thanks to advances in modeling and artificial intelligence, doses will be more precisely delivered and less toxic to immune cells. Combined with a drug that aims to increase the number of lymphocytes, radiotherapy will be less deleterious," explains Prof. Eric Deutsch, Head of the Radiotherapy Department at Gustave Roussy.
This interdisciplinary research program combines the complementary expertise of Gustave Roussy and the Centre Léon Bérard, with that of 3 academic centers: Inserm, ESSEC and the University of Paris Saclay; and an innovative biotech company: Therapanacea (world leader in radiotherapy planning).
4 key pillars based on clinical trials and artificial intelligence
First, a new artificial intelligence-guided digital pathology platform will be developed with the goal of being able to create a 3D histology map of the tumor based on routine CT imaging. This 3D map will then be used to accurately delimit the tumor from the healthy tissue in a non-invasive manner and thus to reduce uncertainties that currently force us to apply non neglectable safety margins.
Second, the LySAIRI consortium will propose new "immunological" dose constraints, which will be used routinely in the future to improve the care of patients receiving radiotherapy.
The third pillar will allow the smooth integration of the digital pathology tool and the immunological dose constraints into the routine clinical software that radiation oncologists use to prescribe radiotherapy. The end product will be a cloud-based radiation treatment planning system that will automatically produce a personalized immune-sparing contouring for the treatment planning of patients requiring radiation therapy.
The final pillar will consist in the clinical validation of the lymphocyte-sparing radiotherapy strategy. A drug will be used in combination with lymphocyte-sparing radiotherapy to ensure the support of anti-tumor immune cells. It will be validated by an in-depth medico-economic analysis, ensuring that the efficiency of the approach developed is assessed to facilitate large-scale adoption of the strategy.
Two clinical trials will be carried out, the first in patients with solid cancers at an oligometastatic stage (presenting a few metastatic tumor lesions, no more than 5), the second in patients with certain locally advanced head and neck cancers. The aim will be to clinically evaluate the appearance, or not, of lymphopenia after the start of radiotherapy, and to correlate this with treatment outcomes and quality of life.
LySAIRI aim and vision