Paris, le 2 février 2022
Cancer Treatment: Identification of the Blood Vessels That Allow Killer Lymphocytes to Access and Destroy Tumors
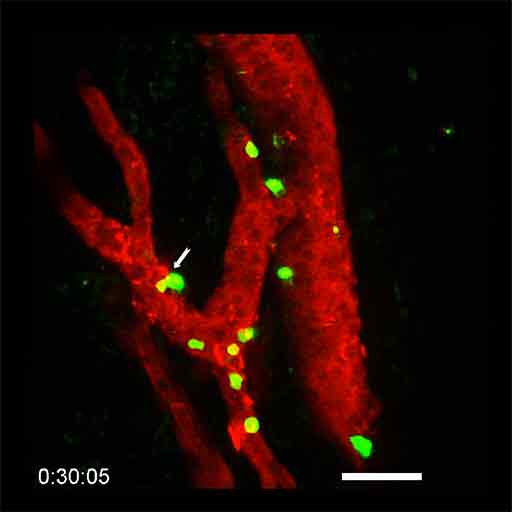
Microscopic visualization of lymphocytes (in green) infiltrating a tumor HEV (in red) during combination anti-PD-1 plus anti-CTLA-4 immunotherapy. The white arrow indicates a lymphocyte that is leaving the bloodstream and entering the tumor (in black). © Elisabeth Bellard and Jean-Philippe Girard - IPBS (CNRS/UT3 Paul Sabatier)
Immunotherapy, a therapeutic strategy aimed at increasing the activity of the immune system in order to recognize and destroy cancer cells, has revolutionized cancer treatment over the past decade. A better understanding of how this therapeutic approach works, and more particularly how killer lymphocytes access tumors during immunotherapy, could improve the efficacy of the treatments. The team of Jean-Philippe Girard, Inserm Research Director at the Institute of Pharmacology and Structural Biology (French National Center for Scientific Research [CNRS]/Université Toulouse III - Paul Sabatier), in collaboration with Gustave Roussy, has recently discovered the essential role played in this process by specific blood vessels known as tumor-associated HEVs. For the first time, the scientists were able to film the lymphocytes infiltrating the walls of the HEV vessels to enter the tumors. What is more, the researchers have shown in animal models that increasing the proportion of HEV vessels in a tumor improves the efficacy of the immunotherapy and leads to the eradication of the tumors. Finally, they found that the likelihood of recovery of patients with metastatic melanoma (skin cancer) and treated with immunotherapy is increased when a large number of HEV vessels are present in tumors. The findings of this study have been published in the February 3, 2022 issue of Cancer Cell1 .
Immunotherapy with therapeutic antibodies represents a real revolution in cancer treatment. In particular, it is used to cure certain patients with metastatic melanoma (skin cancer), who would previously not have survived. Unfortunately, immunotherapy is not effective in all patients or on all cancers. A better understanding of the mechanism of action of the treatment could improve it and make it effective in a larger number of patients.
Killer lymphocytes – white cells present in the blood – are capable of eradicating cancer cells. It is essential that many of these killer cells have access to tumors in order to defend the body against cancer. The Toulouse team has lifted the veil on the mechanisms that allow killer lymphocytes to penetrate tumors in order to destroy them – either spontaneously or following immunotherapy with anti-PD-1 plus anti-CTLA-4 antibodies.
The scientists have discovered that the HEVs, very specific blood vessels known as high endothelial venules, constitute the major gateway of lymphocytes to tumors. Using sophisticated microscopy techniques, the researchers were able to film the passage of lymphocytes from the blood to the tumor in animal models. For the first time, they were able to visualize, directly and in real time, the lymphocytes in the process of infiltrating the walls of the HEV vessels in order to access the cancer cells in the tumor. "We thought that HEV vessels played an important role in the entry of lymphocytes into the tumor, but we were surprised to see that they were virtually the only gateway," says Jean-Philippe Girard, Inserm Research Director and last author of the study.
The researchers then observed in their models that the presence of a large number of killer lymphocytes in tumors is associated with the presence of a large number of HEV vessels. What is more, they provided proof of concept that increasing the proportion of HEV vessels in a tumor improves the efficacy of combination anti-PD-1 plus anti-CTLA-4 immunotherapy and leads to tumor eradication.
Finally, in collaboration with Caroline Robert's team at Gustave Roussy2, the scientists studied patients with metastatic melanoma. They discovered that the presence of a large number of HEV vessels in tumors is associated with a better response to combination anti-PD-1 plus anti-CTLA-4 immunotherapy.
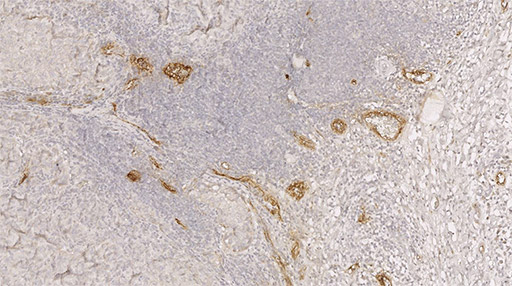
Labeling of HEV vessels (brown) on a tumor section from a patient with metastatic melanoma and treated with immunotherapy. © Jean-Philippe Girard - IPBS (CNRS/UT3 Paul Sabatier)
The next step for the researchers will be to develop treatments to increase the proportion of HEV vessels in tumors, in order to improve the efficacy of immunotherapy, allowing massive recruitment of killer lymphocytes to eradicate cancer cells. "Our research could improve immunotherapy treatment in the longer term for patients with metastatic melanoma and other types of solid tumors. It also has prognostic implications, as clinicians can now look at the HEV vessels to predict a patient's response to immunotherapy," concludes Girard.
1 This study was funded by Fondation ARC, the Foundation for Medical Research (FRM), the French National Cancer Institute (INCa), the French National Research Agency (ANR), and Labex TOUCAN
2 And also team leader at Inserm U981
Sources
“Tumor-associated high endothelial venules mediate lymphocyte entry into tumors and predict response to PD-1 plus CTLA-4 combination immunotherapy”
Assia Asrir1,7, Claire Tardiveau1,7, Juliette Coudert1,7, Robin Laffont1,7, Lucas Blanchard1,7, Elisabeth Bellard1, Krystle Veerman1, Sarah Bettini1, Fanny Lafouresse1, Estefania Vina1, Dorian Tarroux1, Severine Roy2, Isabelle Girault2, Irma Molinaro5, Frédéric Martins3,4, Jean-Yves Scoazec5,6, Nathalie Ortega1, Caroline Robert2,6 and Jean-Philippe Girard1,8*
1 Institut de Pharmacologie et de Biologie Structurale, IPBS, Université de Toulouse, CNRS, UPS, Toulouse, France
2 Department of Medicine, Gustave Roussy, Villejuif, France
3 Institut des Maladies Métaboliques et Cardiovasculaires, I2MC, UMR1048, INSERM, UPS, Toulouse, France
4 Plateforme Genome et Transcriptome, GeT, Genopole Toulouse, France
5 Department of Pathology, Gustave Roussy, Villejuif, France
6 Paris-Saclay University, Orsay, France
7 These authors contributed equally: Assia Asrir, Claire Tardiveau, Juliette Coudert, Robin Laffont, Lucas Blanchard
Cancer Cell, February 2022
DOI: https://doi.org/10.1016/j.ccell.2022.01.002
