Hematopoietic tissue aging
This group belongs to the UMR 1287 - Hematopoietic stem cells and the development of myeloid malignancies
Current research
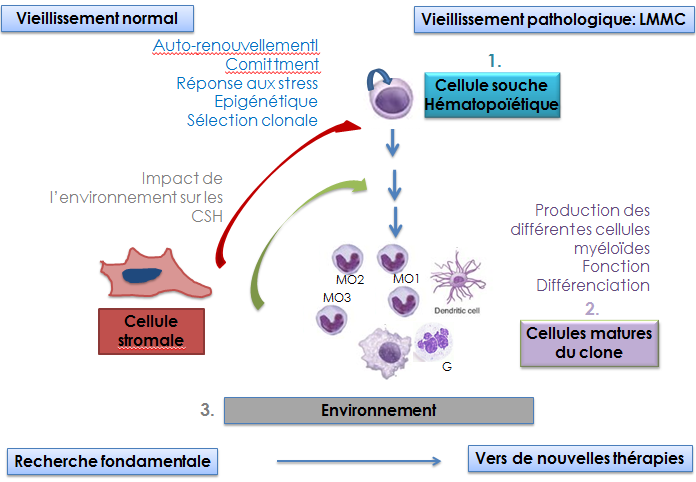
The world's population is aging. This demographic change requires exploration of the pathophysiological effects of aging, one of which is the emergence of cancers. Aging of the hematopoietic tissue, for example, leads to alteration in the composition and function of blood cells, decreases adaptive immunity, and contributes to the emergence of isolated cytopenias and chronic diseases, and predisposes to the development of malignant hematopathies such as myelodysplastic syndromes (MDS), myeloproliferative neoplasms (MPN), and acute myeloid leukemia (AML). Exploring the molecular, cellular and tissue impact of aging should suggest strategies for the prevention and early detection of these diseases.
The team is exploring how aging subverts HSC differentiation towards the myeloid lineage, leading in some cases to clonal expansion. We investigate the molecular mechanisms that drive myelomonocytic expansion during physiological aging in a normal context, during various stresses and in a pathological context: chronic myelomonocytic leukemia (CMML), a model myeloid malignancy because 1) typically associated with aging; 2) associating dysplasia with a proliferative component. We will explore epigenetics or genetic alterations in the HSCs and the mature cells, the impact of the microenvironment on myelomonocytic differentiation, the changes in the nature, function and fate of different populations of mature monocytes, the feedback loops beween these cells and HSCs and whether functional and genetic alterations intrinsic to bone marrow stromal cells or more distant factors such as variations in the composition of the microbiota may be factors driving the clinical expression of CMML. This project is at the interface of different pathologies: cancer, cardiovascular diseases, immunity/inflammation/infection. By exploring the pathophysiology of CMML, it should generate new therapeutic approaches, explore them in preclinical models and transfer them to the clinic, thanks to close links with Gustave Roussy's phase I unit and the medical teams of the groupe Francophone des Myélodysplasies.
Transdisciplinarity enriches our work. We welcome a Philosopher of Science (CNRS researcher) into the team who explores with a different perspective the question of the identity of normal and leukemic HSCs and clonal evolution. We have also launched a clinical trial aimed at establishing in partnership a mathematical model of monocyte production that will generate mechanistic and quantitative hypotheses to guide our biological research.
HSC response to stress and normal and pathological aging (Emilie Elvira-Matelot, Françoise Porteu)
Selection pressures are regulating HSC self-renewal and responses to stress. Aging hematopoiesis may therefore be viewed as the net result of many selection pressures that promote alterations in the composition of HSCs that could lead to the emergence of competitive HSC clones. These clones may be predisposed to oncogenic transformation because they have been selected for their ability to self-renew under genotoxic stress or other stress conditions that occur with aging. Thus, understanding the molecular mechanisms leading to alterations induced by low dose IR is likely to give new insights on HSC behavior during aging and age-associated myeloid malignancies. In recent years, we have shown that the cytokine Thrombopoietin (TPO) increases the fidelity and efficiency of Non-Homologous End Joining (NHEJ) DNA repair pathway in response to genotoxic stress by directly stimulating DNA-PK (de Laval et al., Cell Stem Cell 2013 & Blood 2014). We have demonstrated a new molecular mechanism involved in the premature aging of HSCs: the intervention of retroelements (RE). This response is controlled by TPO via induction of an antiviral response. (Barbieri et al., J Exp Med 2018). Our projects combine fundamental experimental approaches applying inflammatory and genotoxic stresses in mice and human models, as well as the study of HSCs from patients with CMML as a model disease of the aging hematopoietic system to investigate the links between chromatin dynamics, DNA repair, cytokine modifications and signaling in aging HSCs and clonal selection. We are also studying the relationships between retroelements, genomic instability and anti-tumor immunity.
Aging of the monocytic lineage (Nathalie Droin, Dorothée Selimoglu-Buet, Eric Solary)
One of the consequences of the aging of hematopoietic tissue is the degradation of the innate and adaptive immune response (immuno-senescence), in particular due to an alteration in the number and functions of the cells involved. Peripheral blood monocytes represent between 5 and 10% of circulating leukocytes. They are key cells in the innate and adaptive response. One of the key cytokines in monocytopoiesis is CSF1. We have shown that a fraction of its receptor, CSF1R, is located in the nucleus of monocytes and macrophages and participates in transcriptional regulation (Bencheikh et al., Nat Common 2019).
Monocytes are heterogeneous. An international nomenclature distinguishes 3 populations in the blood. Our team has identified changes in the distribution of these populations in CMML patients (Selimoglu-Buet et al. Blood, 2015). We demonstrated that a miR-150 / TET3 pathway, conserved in mice and humans, contributed to the abnormal distribution of circulating monocytes in CMML patients (Selimoglu-Buet et al., Nat Common 2018).
We are studying the impact of aging on the monocytic lineage by investigating how aging of monocytes and macrophages 1) affects the nuclear functions of CSF1R; 2) contributes to changes in the bone marrow niche and affects HSCs, including chromatin conformation, myeloid differentiation and self-renewal in response to stress. We continue to explore monocyte heterogeneity by 1) creating an atlas of monocyte populations in normal and pathological situations; 2) analyzing the versatility of human monocytes; 3) characterizing the functions of newly identified populations; 4) identifying the molecular mechanisms governing monocyte heterogeneity.
Pathophysiology and treatment of CMML (Eric Solary, Nathalie Droin, Dorothée Selimoglu-Buet).
We have explored the limits of the efficacy of hypomethylating agents, demonstrating that these molecules restore balanced hematopoiesis but do not eliminate genetic alterations or prevent the genetic evolution of the clone and the ultimate transformation of the chronic disease into acute leukemia (Merlevede et al., Nat Commun 2016). These results highlight the importance of epigenetic alterations in the clinical-biological expression of the disease and the need to identify alternative therapeutic approaches to eradicate the leukemic clone. We have modeled CMML in vitro by reprogramming CD34+ cells from patients into stem cells (Beke et al., Haematologica 2020), and in vivo in a xenograft model of CMML patient cells in immunocompromised mice (Zhang et al., Blood Advances 2018). We will continue to study the pathophysiology of CMML, searching for therapeutic solutions by exploring 1) the contribution of recurrent mutations of DNA coding regions to the clinical and biological expression of CMML, in particular by generating genetically modified mouse models; 2) the role of mature cells of the leukemic clone and their immunosuppressive and pro-inflammatory effects; 3) the contribution of alterations in the hematopoietic niche to the onset or progression of CMML.
Philosophy of Science (Lucie Laplane)
We believe that philosophy can contribute to science through conceptual analysis (Laplane et al. PNAS 2019). We are developing 3 lines of research associated with the team's experimental research: stem cells, tumor microenvironment and clonal evolution. The somatic genetic events that characterize the leukemic clone in CMML accumulate in the stem cell compartment. These cells, which could be termed "leukemic stem cells", appear to be the best therapeutic target within the clone. Our work indicates that a stem cell can fall into four categories depending on whether the stem cell property is intrinsic or extrinsic, dependent or independent of the environment. The category to which a stem cell belongs may evolve over the course of the disease and require different therapeutic strategies (Laplane and Solary., Elife. 2019). The identity and quantity of leukemic stem cells may impact clonal dynamics (Laplane, Biology and Philosophy 2019). We are seeking to better characterize and integrate the different evolutionary processes at work in clonal evolution, during aging (clonal hematopoiesis) and malignant transformation, particularly in CMML (genetic & epigenetic evolution, cell dynamics). Finally, the microenvironment is increasingly central to cancer and stem cell biology. Nevertheless, the spatial boundaries of the microenvironment are blurred and the concept covers heterogeneous causal contributions that we are seeking to better characterize (Laplane et al., Int J Cancer. 2019; Trends in Cancer 2018).